The process defined by the new German DVG regulation for the reimbursement of digital health solutions paves the way for German leadership in the European digital health market, pointing a way forward also for the other EU countries!
Germany position as the forerunner became official on November 7th, 2019, when its Parliament passed the Digital Health Service Act (Versorgung-Gesetz – DVG) aimed at encouraging the use of digital health applications for patients, and providing for a reimbursement mechanisms set out in the Fifth Social Security Code.
According to official projections, as of 2020 more than 73 million Germans will be eligible and for health costs reimbursed by the public health system, and now this also covers digital apps for therapeutic purposes.
90% of the German population is covered by public health insurance, while the remainder relies on private packages that, most likely, will also fall in-line to provide the same levels of coverage for digital health solutions.
This ambitious process was started by German Government Health Minister Jens Spahn, in close collaboration with the Health Innovation Hub (HIH), a task force mandated to “rethink health” from a digital perspective. That effort was led by Dr. Henrik Matthies, who has a strong entrepreneurial background in the field of health.
Which solutions will be eligible for reimbursement?
According to the new regulation, coverage will be extended to digital health solutions similar to low risk class medical devices (class I or IIa) with functionalities mainly based on digital technologies (apps or software solutions). To be eligible for reimbursement, the solution must be used for the diagnosis, monitoring and treatment of diseases or to improve the quality of treatment.
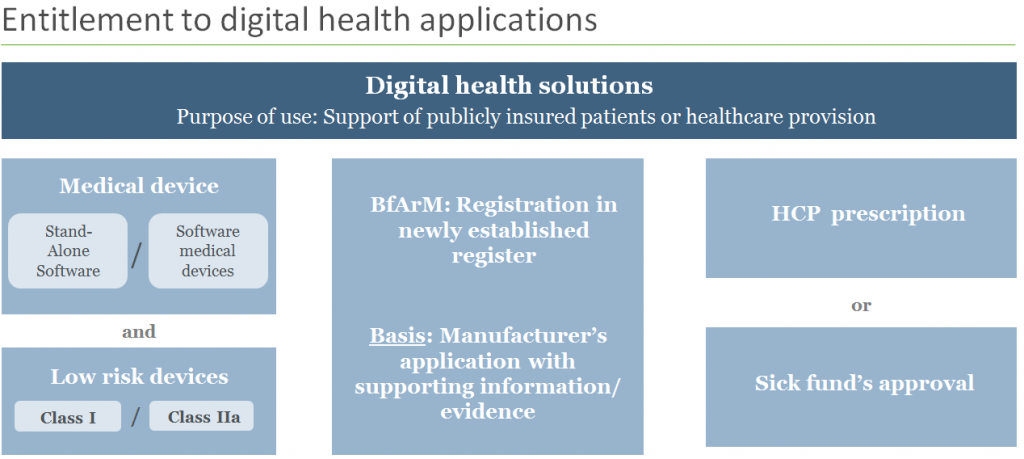
Entrepreneurs can submit applications for their digital health solutions for pre-approval and through a fast-track process. This opens the door to a 12 months in-market trial period for those solutions, during which time they will be subject to thorough testing and data collection of their efficacy.
What providers must guarantee?
Not only must safety, functionality, quality and adherence to data protection rules be demonstrated, but the manufacturer must also provide reasonable reasons (scientific evidence is not yet required at this stage) for the contribution that the product will make in terms of improving the process of patient care, quality of life and ethical and legal protections.
If the solution can prove its effectiveness in the 12 months in-market, the federal body (GKV-Spitzenverband) and the manufacturers of the solution will enter negotiations on the amount of be reimbursed for its continued presence in market.
As Dr. Henrik Matthies said, on the occasion of his deep dive at the conference Frontiers Health 2019, last November 14 in Berlin, it will take about three months to decide on the application for the inclusion of a “dam” (digital application) in the health system, so entrepreneurs involved can see the first applications accepted in the system towards the second quarter of 2020. As Matthies also pointed out, the German government has allocated a budget of 200 million euros for 2020 which will provide the pool of funds available for the first wave of digital solutions approved in the health system.
Conclusions
The regulatory framework is in its early stages and aims to be improved. Also important to note that the reimbursement only covers Class I and IIa software medical devices, while non-medical devices or digital apps combined with other medical devices are not covered by the regulation. In addition, the requirements for entering the dedicated register are very stringent. The new regulations are also testimony to a clear ambition for Germany to have political leadership (Germany’s health system being the largest in Europe) and commercial leadership, with it also being the largest healthcare market after the United States. The German government intends to play this role to the fullest extent, making the most of their Presidency of the European Semester, July-December 2020, which also opens the door for Germany to propose and launch an initiative on health data management across all of Europe.
As Germany has taken the initiative to show the way, we can only hope for a spill-over effect on all the other EU countries, and the benefits that EU citizens can gain from this welcome change.



