An innovative study conducted at the Harold Schnitzer Diabetes Health Center clinic at Oregon Health and Science University has demonstrated that integrating real-time wearable fitness data with automated insulin delivery (AID) systems can significantly improve glucose control and enhance the safety of exercise for individuals with type 1 diabetes.
The research, funded by the JDRF Foundation, the Leona M and Harry B Helmsley Charitable Trust, and the National Institutes of Health, marks a significant advancement in diabetes management.
Background: People with type 1 diabetes face unique challenges when engaging in physical activities like exercise due to the potential for dangerous fluctuations in blood glucose levels. While exercise is known to rapidly lower glucose levels, there has been a lack of integration between ubiquitous wearable fitness sensors and AID systems. This study aimed to test whether AID systems could use real-time fitness data to automate insulin adjustments, reducing the risk of hypoglycemia during exercise and daily life.
Methodology
The study included individuals who met specific inclusion criteria, such as having a diagnosis of type 1 diabetes for at least 1 year, being between the ages of 21 and 50, being physically capable of aerobic exercise, using an insulin pump for at least 3 months with stable settings for 2 weeks, and having a baseline HbA1c level of 10·0% or lower. Additionally, participants needed to live with an adult aged 18 years or older within 40 miles of OHSU and have a total daily insulin requirement of less than 139 units per day to ensure that meal insulin could be delivered within 20 minutes.
It commenced with a screening visit conducted within 12 weeks before a 1-week run-in period. During this visit, participants provided written informed consent, underwent eligibility screening, and had their HbA1c levels measured. Additionally, an electrocardiogram (EKG) was performed. Once eligibility was confirmed, participants engaged in a 1-week run-in period during which they received training on using the Dexcom G6 continuous glucose monitoring system (CGM). Subsequently, participants arrived at the OHSU inpatient research unit to initiate the first 76-hour treatment phase.
Participants were assigned to use either an AID that detects exercise, prompts the user, and adjusts insulin during exercise using an exercise-aware adaptive proportional derivative (exAPD) algorithm or an AID that automates insulin adjustments using fitness data in real-time through an exercise-aware model predictive control (exMPC) algorithm. Both algorithms ran on an iPancreas system, incorporating commercial glucose sensors, insulin pumps, and smartwatches.
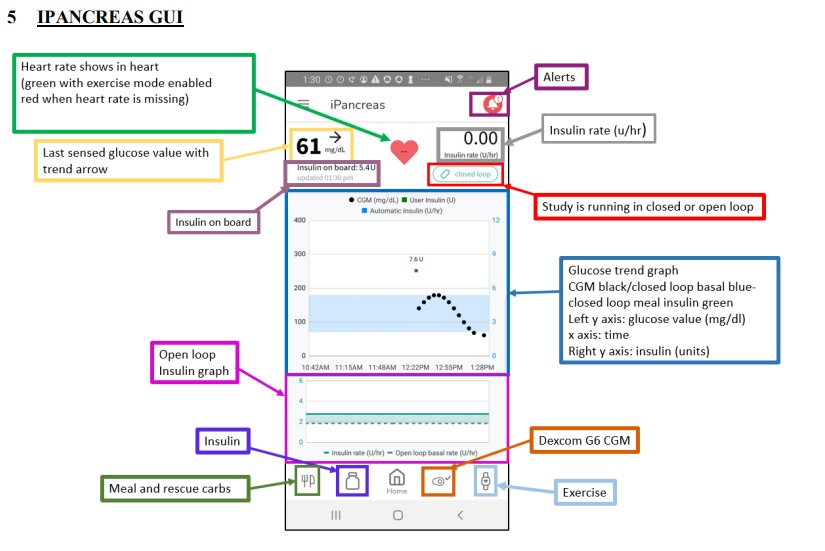
Overview of iPancreas Test Platform
iPancreas is an innovative and adaptable system designed to facilitate the swift development of closed-loop and decision support algorithms, as well as user interfaces for effective glucose management. This system is modular, licensable, and available for open access, offering researchers and developers a versatile tool for prototyping glucose management solutions.
The iPancreas system comprises several key components, including:
- Custom iPancreas App: The system operates through a custom-designed application that runs on a Samsung smartphone from Suwon-Si, Korea. This app serves as a central control hub for managing glucose levels and insulin delivery.
- Dexcom G6 CGM: The system integrates a Dexcom G6 continuous glucose monitoring (CGM) system, which wirelessly provides real-time glucose data to the smartphone.
- Research Version Insulet Omnipod: An Insulet Omnipod, designed for research purposes, is included in the system. It is responsible for insulin delivery based on the control algorithm’s calculations.
- Polar M600 Smartwatch: The iPancreas setup includes a Polar M600 smartwatch from Kempele, Finland. This smartwatch captures essential data such as heart rate and accelerometry, which can be valuable for monitoring physical activity and its impact on glucose levels.
- Custom-Developed Cloud Monitoring and Data Acquisition Repository: To manage and analyze the data generated by the system, a custom-developed cloud-based monitoring and data acquisition repository is employed. This repository runs on Amazon Web Services (AWS) in Seattle, WA, USA, ensuring efficient data handling and storage.
The core functionality of iPancreas involves wirelessly receiving CGM data and data from the Polar M600 smartwatch through the smartphone. The system’s control algorithm processes this data to calculate the appropriate insulin dose, which is then transmitted wirelessly to the Insulet Omnipod for insulin delivery. Additionally, iPancreas incorporates a meal bolus calculator to assist users in managing their insulin dosage in response to meals.
Overall, iPancreas offers a versatile and comprehensive platform for the development and testing of glucose management solutions, with the potential to advance diabetes care and treatment options.
Outcomes
The time spent below the target glucose range (39 mmol/L) during the primary in-clinic session between April 13, 2021 and October 3, 2022 was compared in the research. There was no significant difference in time spent below the target range between the exMPC and exAPD groups. The exMPC group, on the other hand, had lower mean glucose levels after two hours of in-clinic activity. Both algorithms met clinical time in range objectives, with exMPC achieving 71.2% and exAPD achieving 75.5%. However, as compared to the run-in period, the exMPC group dramatically reduced time spent below the goal range. During the trial, no adverse events were noted. Secondary outcome measures included determining the amount of time spent within the target glucose range, the amount of time spent over the goal range, and the number of rescue carbs required each day in reaction to hypoglycemia.
Conclusions
The study demonstrated that both exMPC and exAPD algorithms, which incorporate exercise metrics, yield similar glucose outcomes in individuals with type 1 diabetes.
During the primary in-clinic session, both algorithms showed comparable time spent in the target glucose range and time below the target range. However, the exMPC algorithm outperformed the exAPD algorithm in the two hours following the start of structured exercise, significantly lowering mean glucose levels without a significant increase in time spent below the target range or experiencing very low glucose.
This represents a pioneering effort in integrating exercise metrics as continuous inputs into an automated insulin delivery system to modify insulin dosing under real-world, free-living conditions. The exMPC algorithm could adapt to exercise events throughout the day, including activities of daily living. Both algorithms effectively maintained participants above 70% of the time within the target glucose range and kept time spent below the target range below 4%, aligning with recommendations from the American Diabetes Association.
However, the study has several limitations, including a relatively short duration, limited evaluation of structured exercise, potential noise introduced by the FDA’s requirement for an in-clinic evaluation, and the specificity of the results to one type of fitness watch, the Polar M600. Further research is needed to address these limitations and validate the findings in broader contexts.